You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Coronavirus good news thread
- Thread starter JM14
- Start date
bear66
Well-known member
They're very efficient there.Vaccine booked for tomorrow. Got really lucky with the dates. Tried this morning and they could only offer me Fridays at the end of the month. Not suitable for me so tried again this afternoon and gotnam appointment in acklam tomorrow morning.
Randy
Well-known member
Will I get a cup of tea?They're very efficient there.
bear66
Well-known member
No time! 4 minutes from arriving to leaving.Will I get a cup of tea?
Randy
Well-known member
Disappointed. The website said it was a 30-40 minute appointment, assumed that included refreshments and biscuits like you get a blood donors appointment.No time! 4 minutes from arriving to leaving.
NorthumberlandBoro
Well-known member
Had my second at my GPs at the weekend. No queue straight in, few q's, sat down, jabbed and out.
Doubt it even took 2 minutes.
Nowt but a sore arm afterwards.
Doubt it even took 2 minutes.
Nowt but a sore arm afterwards.
Randy
Well-known member
Jonny_Rondos_Disco_pants
Well-known member
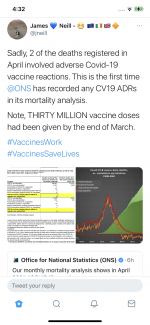
Now a bit of good/bad news, depends how you want to look at it.
2 deaths reported involving vaccines have been declared on death certs to the ONS. They were not the direct cause of death which is good news.
30m doses given, seems like justified risk to me.
2 deaths reported involving vaccines have been declared on death certs to the ONS. They were not the direct cause of death which is good news.
30m doses given, seems like justified risk to me.
Attachments
A new study shows that immunity from a naturally-acquired Covid-19 infection can last for up to a year and is significantly boosted by a subsequent vaccination.
Vaccination boosts naturally enhanced neutralizing breadth to SARS-CoV-2 one year after infection
Extracts from the paper:
Vaccination boosts naturally enhanced neutralizing breadth to SARS-CoV-2 one year after infection
Extracts from the paper:
neutralizing [antibody] titers remain relatively unchanged between 6 to 12 months after SARS-CoV-2 infection, and [...] vaccination further boosts this activity by nearly 50-fold.
In the absence of vaccination, the number of RBD specific memory B cells present at 12 months was only 1.35-fold lower than the earlier timepoint (p= 0.027, Fig. 2b). In contrast and consistent with previous reports, convalescent individuals that received mRNA vaccines showed an average 8.6-fold increase. in the number of circulating RBD specific memory B cells.
Last edited:
Novavax's two-dose COVID-19 vaccine had an overall efficacy of 90%, with 100% efficacy against moderate and severe disease, the manufacturer announced on Monday.
Another highly effective Covid vaccine
In a bit of, "it almost seems too good to be true" news, the manufacturer's announcement gives preliminary data that it may be even more effective against variants of concern:
Another highly effective Covid vaccine
In a bit of, "it almost seems too good to be true" news, the manufacturer's announcement gives preliminary data that it may be even more effective against variants of concern:
An exploratory analysis involving sequenced cases of variants of concern or interest -- 38 in the placebo group and six in the vaccine group -- demonstrated a vaccine efficacy of 93.2%.
Last edited:
An interesting side-note perhaps (at least I find this kind of stuff fascinating) is that this vaccine uses a different technology to any of the currently authorised vaccines - it's a prefusion, protein nano-particle sub-unit vaccine construct.
It seems to be another indication that using the prefusion form of the spike protein may give better results than the postfusion form.
According to the research, the SARS-CoV-2 spike protein changes shape after it attaches to a human host cell.
For instance, the Pfizer-BioNTech and Moderna vaccines also use the prefusion form, while the Oxford-AstraZeneca vaccine uses the postfusion form.
As the article below points out, this makes sense - you want the antibodies and cytotoxic T cells to attack the virus before it infects the host cell, not after.
The tiny tweak behind COVID-19 vaccines
It seems to be another indication that using the prefusion form of the spike protein may give better results than the postfusion form.
According to the research, the SARS-CoV-2 spike protein changes shape after it attaches to a human host cell.
For instance, the Pfizer-BioNTech and Moderna vaccines also use the prefusion form, while the Oxford-AstraZeneca vaccine uses the postfusion form.
As the article below points out, this makes sense - you want the antibodies and cytotoxic T cells to attack the virus before it infects the host cell, not after.
The tiny tweak behind COVID-19 vaccines
FabioPorkpie
Well-known member
Another drug to add to the weaponry, confirmed by the U.K. based Recovery trial.
‘It found that the antibody therapy reduced by a fifth the 28-day mortality of people admitted to hospital with COVID-19 whose immune system had not mounted an antibody response’
‘The treatment also shortened the hospital stay of those who were seronegative and reduced their chances of needing a mechanical ventilator, Landray said.’
"This is the first time we've got one that's actually targeting the virus itself," Landray said, adding that it could be used along with the other treatments.
"It's not that you do one thing or another thing. These benefits are additive in these patients," he said.
/cloudfront-us-east-2.images.arcpublishing.com/reuters/OCXS2BK2VFOSVMECV7TI45TTQQ.jpg)
 www.reuters.com
www.reuters.com
‘It found that the antibody therapy reduced by a fifth the 28-day mortality of people admitted to hospital with COVID-19 whose immune system had not mounted an antibody response’
‘The treatment also shortened the hospital stay of those who were seronegative and reduced their chances of needing a mechanical ventilator, Landray said.’
"This is the first time we've got one that's actually targeting the virus itself," Landray said, adding that it could be used along with the other treatments.
"It's not that you do one thing or another thing. These benefits are additive in these patients," he said.
/cloudfront-us-east-2.images.arcpublishing.com/reuters/OCXS2BK2VFOSVMECV7TI45TTQQ.jpg)
Regeneron’s antibody therapy cuts deaths among some hospitalised COVID-19 patients -study
A COVID-19 antibody cocktail developed by Regeneron Pharmaceuticals Inc (REGN.O) and Roche (ROG.S) reduced deaths in hospitalised patients whose own immune systems had failed to produce a response, a large British study found on Wednesday.
bear66
Well-known member
Listening to a researcher this morning, it isn't normal to measure patients' antibody response on admission with covid. The death rate of those with no antibody response is double that of those that have some antibody response. This seems to be an important marker that will presumably become a normal test in future.Another drug to add to the weaponry, confirmed by the U.K. based Recovery trial.
‘It found that the antibody therapy reduced by a fifth the 28-day mortality of people admitted to hospital with COVID-19 whose immune system had not mounted an antibody response’
‘The treatment also shortened the hospital stay of those who were seronegative and reduced their chances of needing a mechanical ventilator, Landray said.’
"This is the first time we've got one that's actually targeting the virus itself," Landray said, adding that it could be used along with the other treatments.
"It's not that you do one thing or another thing. These benefits are additive in these patients," he said.
/cloudfront-us-east-2.images.arcpublishing.com/reuters/OCXS2BK2VFOSVMECV7TI45TTQQ.jpg)
Regeneron’s antibody therapy cuts deaths among some hospitalised COVID-19 patients -study
A COVID-19 antibody cocktail developed by Regeneron Pharmaceuticals Inc (REGN.O) and Roche (ROG.S) reduced deaths in hospitalised patients whose own immune systems had failed to produce a response, a large British study found on Wednesday.www.reuters.com
It's good news that we have additional confirmation that this drug has a beneficial effect but it's not quite a brand new revelation.
This is a drug that was granted an EUA last year and used for instance on Donald Trump when he was hospitalised last November.
What is Regeneron? The drug used in Donald Trump's COVID-19 treatment
This is a drug that was granted an EUA last year and used for instance on Donald Trump when he was hospitalised last November.
What is Regeneron? The drug used in Donald Trump's COVID-19 treatment
bigbob
Well-known member
NYboro
Well-known member
Just started advertising it on TV here......
FabioPorkpie
Well-known member
Another treatment looking like it might be very useful, especially given it is a pill so easy to administer -
‘Molnupiravir is the first oral direct-acting antiviral shown to be “highly effective” at reducing nasopharyngeal SARS-CoV-2 infectious virus and viral genetic material, the researchers at the University of North Carolina and Chapel Hill and Ridgeback Biotherapeutics said in their paper.’
‘Among 202 participants involved in the clinical trial, virus isolation was significantly lower among participants who received 800mg molnupiravir compared with participants who received a placebo used in drug trials to measure effect of experimental therapies. On day 5, the virus was not isolated from any participant who had received 400mg or 800mg molunpiravir — which essentially means they tested negative — compared with 11 per cent of those who had received the placebo.’
The more treatments we can find, the quicker we can open up fully and still have the ability to treat vaccine breakthrough infections, and the unvaccinated.

 www.telegraphindia.com
www.telegraphindia.com
‘Molnupiravir is the first oral direct-acting antiviral shown to be “highly effective” at reducing nasopharyngeal SARS-CoV-2 infectious virus and viral genetic material, the researchers at the University of North Carolina and Chapel Hill and Ridgeback Biotherapeutics said in their paper.’
‘Among 202 participants involved in the clinical trial, virus isolation was significantly lower among participants who received 800mg molnupiravir compared with participants who received a placebo used in drug trials to measure effect of experimental therapies. On day 5, the virus was not isolated from any participant who had received 400mg or 800mg molunpiravir — which essentially means they tested negative — compared with 11 per cent of those who had received the placebo.’
The more treatments we can find, the quicker we can open up fully and still have the ability to treat vaccine breakthrough infections, and the unvaccinated.

Covid: Molnupiravir proves 'highly effective' in reducing viral loads
US-based researchers said the findings demonstrate the safety and tolerability and antiviral efficacy to reduce the replication and accelerate the clearance of SARS-CoV-2
FabioPorkpie
Well-known member
Novavax phase 3 data released and its good news. Another vaccine soon to be added to the arsenal, and this one produced locally 
‘The final analysis confirmed an overall efficacy of 89.7% with over 60% (half) of the cases caused by the B.1.1.7 (Alpha) variant, and a 96.4% efficacy against non-B.1.1.7 (non-Alpha) variants which represents strains most similar to the original virus. ‘
‘We continue to be very encouraged by data showing high levels of efficacy against even mild disease, and that NVX-CoV2373 offers strong cross-protection against both the B.1.1.7 (Alpha) variant and non-B.1.1.7 (non-Alpha) variant strains which are widely circulating," said Gregory M. Glenn, M.D., President of Research and Development, Novavax.’

 www.prnewswire.co.uk
www.prnewswire.co.uk
‘The final analysis confirmed an overall efficacy of 89.7% with over 60% (half) of the cases caused by the B.1.1.7 (Alpha) variant, and a 96.4% efficacy against non-B.1.1.7 (non-Alpha) variants which represents strains most similar to the original virus. ‘
‘We continue to be very encouraged by data showing high levels of efficacy against even mild disease, and that NVX-CoV2373 offers strong cross-protection against both the B.1.1.7 (Alpha) variant and non-B.1.1.7 (non-Alpha) variant strains which are widely circulating," said Gregory M. Glenn, M.D., President of Research and Development, Novavax.’

Novavax Publishes Results of United Kingdom Phase 3 Clinical Trial in New England Journal of Medicine, Demonstrating High Levels of Efficacy of COVID-19 Vaccine
/PRNewswire/ -- Novavax, Inc. (Nasdaq: NVAX), a biotechnology company developing next-generation vaccines for serious infectious diseases, today announced the...
FabioPorkpie
Well-known member
Antiviral Nasal spray with impressive results -

 www.google.co.uk
www.google.co.uk

Made-in-Israel anti-viral nasal spray found effective against COVID
Enovid, a spray developed in Canada by SaNOtize and manufactured in Israel has been found to reduce viral loads in confirmed COVID-19 cases by 95% in 24 hours and 99% in 72 hours
Jostler
Well-known member
Let’s hope testing re Delta is effective and that it’s affordable and readily available, it could be a game changerAntiviral Nasal spray with impressive results -

Made-in-Israel anti-viral nasal spray found effective against COVID
Enovid, a spray developed in Canada by SaNOtize and manufactured in Israel has been found to reduce viral loads in confirmed COVID-19 cases by 95% in 24 hours and 99% in 72 hourswww.google.co.uk